Deuterated Chemistry in Drug Development: A Technical Perspective
GBS
9/20/2025
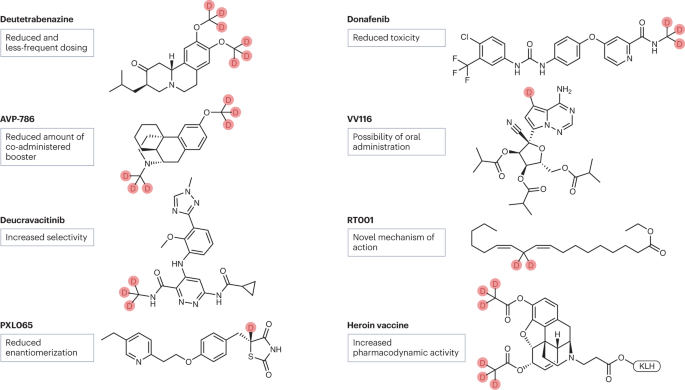
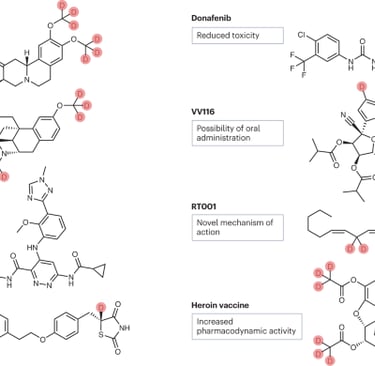
Introduction
Deuterium, a stable isotope of hydrogen, has gained significant attention in drug discovery as a strategic tool to modulate pharmacokinetics (PK) without altering pharmacodynamics (PD). The substitution of hydrogen with deuterium leverages the kinetic isotope effect (KIE), slowing enzymatic metabolism and thereby improving drug exposure, safety, and dosing convenience. Over the past decade, several deuterated drugs have progressed into clinical development and regulatory approval, demonstrating the translational potential of this approach.
Chemical Basis of Deuteration
The core rationale for deuteration lies in the kinetic isotope effect, which arises from the stronger C–D bond (bond dissociation energy ~105 kcal/mol) compared to the C–H bond (~99 kcal/mol). This reduces the rate of bond cleavage by metabolic enzymes such as cytochrome P450s.
Key considerations in deuteration strategies include:
Site-Selective Deuteration: Targeting metabolically labile positions to reduce unwanted biotransformation.
Synthesis Approaches:
Catalytic H/D exchange using transition metals or acids/bases
Deuterated building blocks in stepwise synthesis
Enzymatic or chemoenzymatic incorporation for high selectivity
Deuteration is particularly effective for drugs where oxidative metabolism at specific sites generates toxic or rapidly cleared metabolites.
Pharmacokinetic and Pharmacodynamic Implications
Improved Half-Life: Slower metabolic clearance can extend systemic exposure and reduce dosing frequency.
Reduced Toxicity: By minimizing formation of reactive metabolites, deuteration can improve the safety profile, particularly for drugs with narrow therapeutic windows.
Optimized Dosing: Deuterated analogs can achieve comparable efficacy at lower or less frequent doses.
Figure Suggestion: A schematic comparing normal vs. deuterated drug metabolism and plasma concentration-time profiles.
Case Studies
Drug Indication Deuteration Benefit Status Deutetrabenazine Huntington’s disease, tardive dyskinesia Reduce d dosing frequency, improved tolerability FDA-approved (2017) SD-809 / Teva CNS disorders Increased half-life vs. tetrabenazine Clinical development Other oncology candidates Various PK optimization, reduced hepatotoxicity Preclinical/Phase I
Insights: Deutetrabenazine exemplifies how deuteration can enhance PK/PD without modifying the drug’s mechanism of action. Preclinical studies also show potential in oncology, CNS, and metabolic diseases.
Challenges and Limitations
Variable Effectiveness: The magnitude of KIE is substrate-dependent; not all compounds show meaningful PK improvement.
Regulatory Considerations: Deuterated drugs are treated as new chemical entities by regulatory agencies, necessitating comprehensive safety and efficacy evaluation.
Synthetic Complexity and Cost: Incorporating deuterium may require specialized reagents, catalysts, or multi-step syntheses, impacting scalability.
Intellectual Property: While deuteration can extend patent life, careful IP strategy is required to ensure novelty.
Future Perspectives
Integration with Medicinal Chemistry Strategies: Deuteration can complement prodrug design, targeted delivery, and isotopic labeling for PK/PD studies.
Broader Therapeutic Applications: Oncology, CNS disorders, and metabolic conditions are active areas for development.
Combination Therapies: Deuteration may mitigate drug–drug interactions and enhance combination regimens.
Figure Suggestion: Pipeline illustration showing approved, clinical, and preclinical deuterated drug candidates.
Conclusion
Deuteration represents a subtle yet impactful chemical modification capable of enhancing PK properties, reducing metabolic liabilities, and offering novel intellectual property opportunities. Its increasing application in clinical candidates underscores its value in modern drug discovery, demonstrating how even the substitution of a single neutron can redefine therapeutic potential.
References / Suggested Reading
Gant, T. G. Using Deuterium in Drug Discovery: Leaving the Label Behind. J. Med. Chem. 2014, 57, 3595–3611.
Kratochwil, N. A., et al. Deuterium in Drug Development: Advances and Prospects. Drug Metab. Dispos. 2020, 48, 923–935.
Teva Pharmaceuticals. Deutetrabenazine (Austedo) FDA Approval Summary. 2017.
